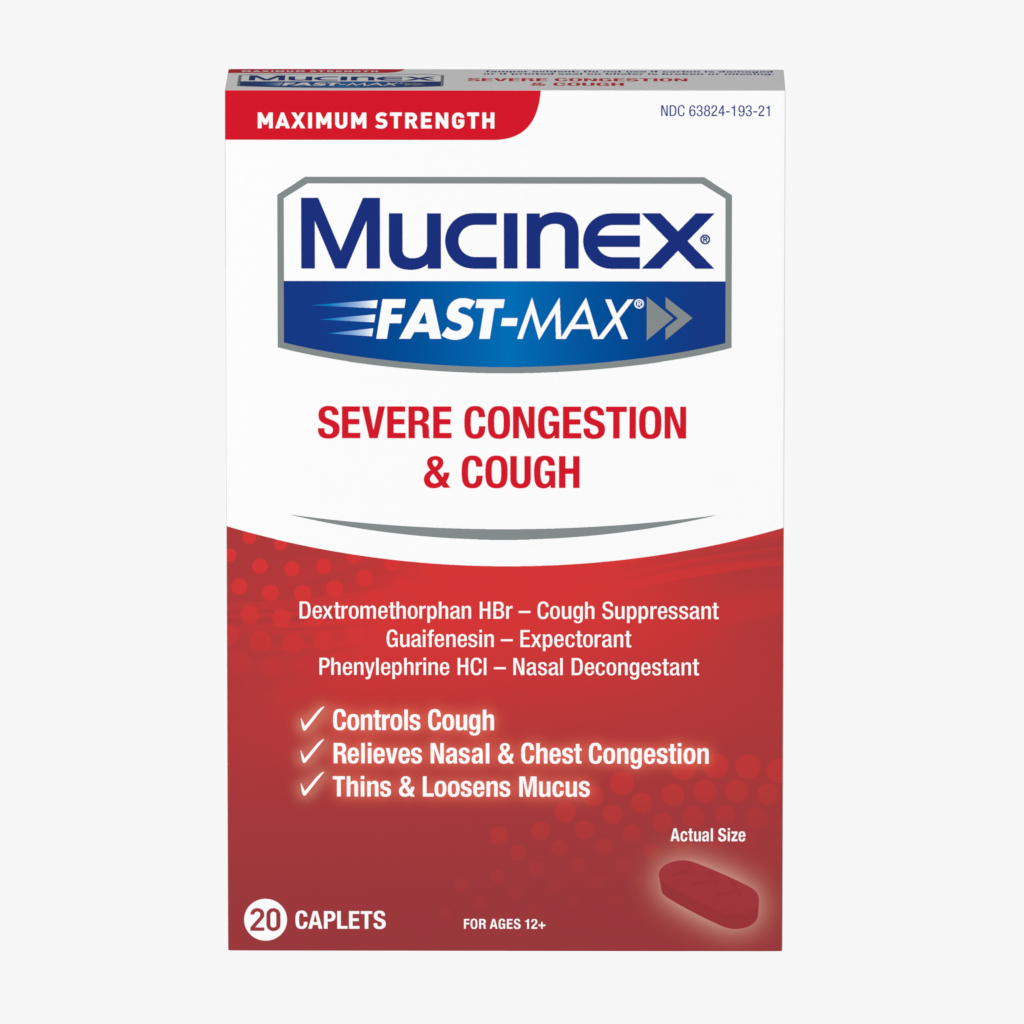
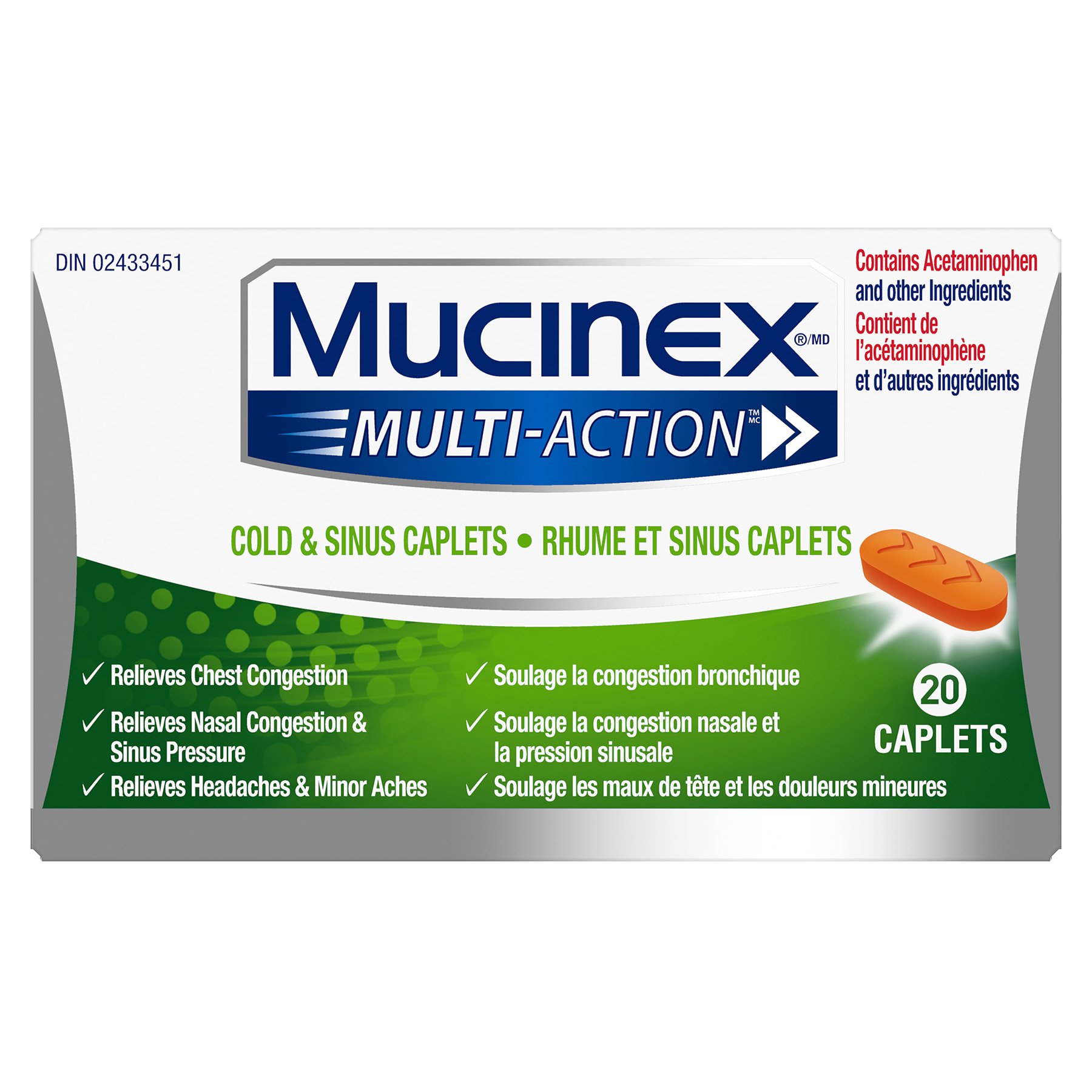
Product
| UPC | Size | Country of Sale |
|---|---|---|
| 3-63824-05718-7 | 18 count box | USA |
| 3-63824-05736-1 | 36 count box | USA |
Items that are grayed out indicate discontinued UPCs.
Ingredient Information
Ingredient |
Function |
|---|---|
| Guaifenesin | DrugA substance that has a physiological effect when ingested or otherwise introduced into the body. |
| Pseudoephedrine HCl | Nasal DecongestantThe active in a drug product used to treat relieve nasal congestion. |
| Microcrystalline Cellulose, NF | ExcipientAn inactive substance that serves as the vehicle or medium for a drug or other active substance. |
| Hypromellose USP | BinderHelps bind the product ingredients together in a tablet format. |
| Sodium Starch Glycolate, NF | DisintegrantA substance used to ensure that solids disintegrate properly after consumption. |
| Carbomer Homopolymer type B | OrganicIngredient or substance that contains carbon atoms. |
| Magnesium Stearate | Processing AidAn ingredient used to increase efficiency or aid in the manufacturing of the product. |
| FD&C Yellow No. 6 Aluminum Lake | ColorantChanges the color of a product (includes dyes and pigments). |
The list above contains the product ingredients listed in descending weight percent. The ingredient names use the INCI (or equivalent) nomenclature system. Click here for more information on the nomenclature system.
Regulatory Information
| Agency | Reg # | Category |
|---|
 United States
United States Canada
Canada